Driving Measurable Outcomes in Cranial Remolding with Sprout3D

Recently recognized as Best in Show – New Product at the 2025 American Orthotic and Prosthetic (AOPA) National Assembly, Sprout3D is reshaping how we approach cranial remolding. At the conference, we shared new evidence on how it addresses the common issues parents and clinicians face with traditional cranial remolding orthoses (CROs). Nuisances such as sweating, skin irritation, poor fit, and weight of the CRO impact the patient and caregiver experience and deter compliance.
We created Sprout3D to address those issues directly. Designed for comfort, ease of use, and quick turnaround, the outcomes speak for themselves. Let’s look at two case studies that show how Sprout3D benefits clinicians, parents, and most importantly, the patient.
Case Study 1: Severe Plagiocephaly
A five-month-old patient presented with severe plagiocephaly, including notable facial asymmetry, as you can see from the initial scans below.
Initial Scan
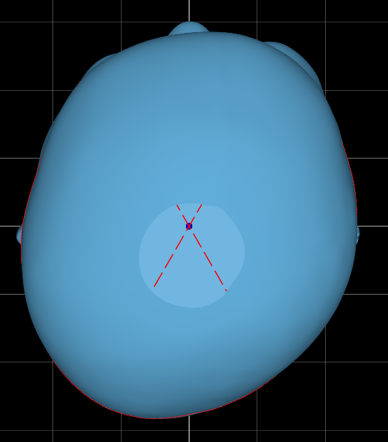
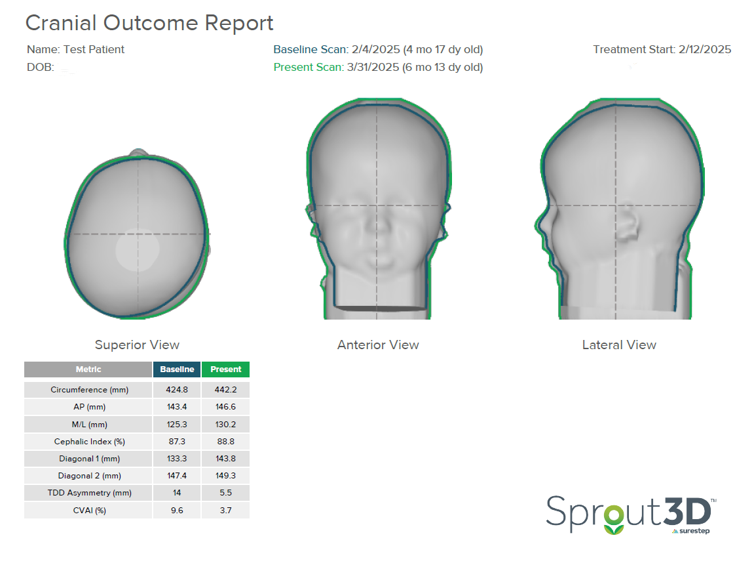
In this patient’s initial outcome report, you can see the severity in the superior transverse plane view of the head. They were scanned at four and a half months and began treatment at five months.
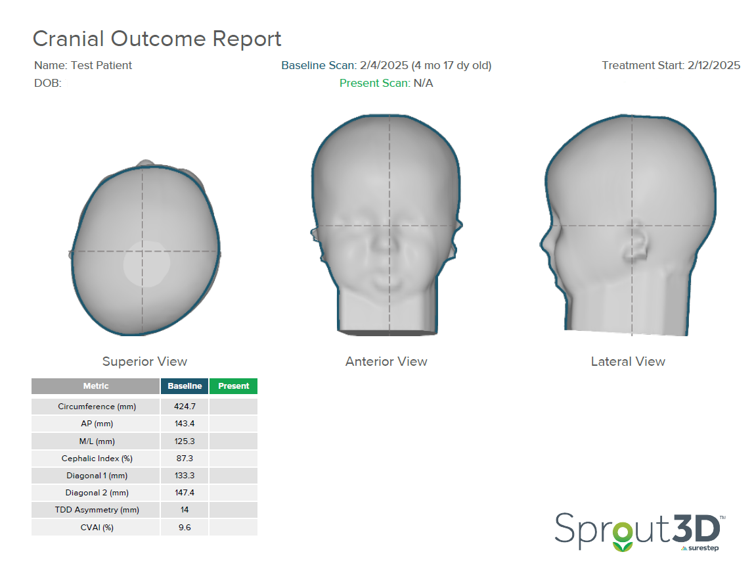
This patient started treatment with a TDD of 14mm and CVAI of 9.6. With a CVAI of 9.6, this patient falls into Level 4 of the Children’s Healthcare of Atlanta Plagiocephaly Severity Scale. There is definite three-quadrant involvement; severe posterior quadrant flattening, possible ear shift, and anterior involvement. Based on this assessment, the treatment recommended by the CHOA Plagiocephaly Severity Scale is a CRO.
Treatment Plan
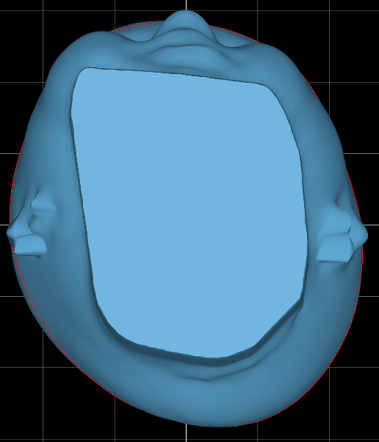
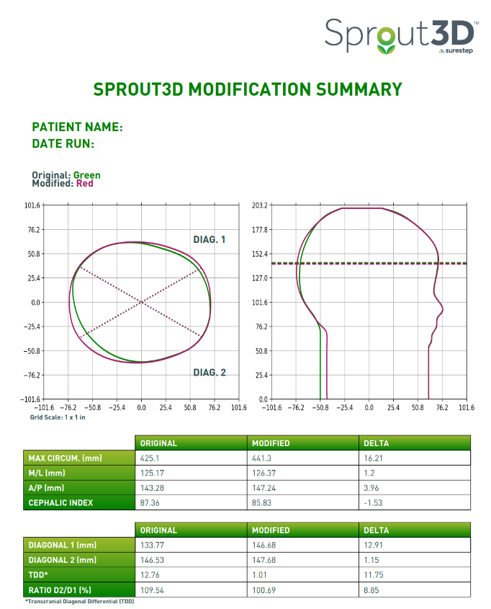
The patient was fitted with their CRO eight days after this scan was taken. This clinician selected standard plagiocephaly modifications for this patient, which means that we build the head as close to full symmetry as possible without exceeding two centimeters or 20 millimeters of circumferential build.
Every Sprout3D comes with the modification summary below, so clinicians have a blueprint for treatment. It provides an opportunity to anticipate where they may experience redness or movement.
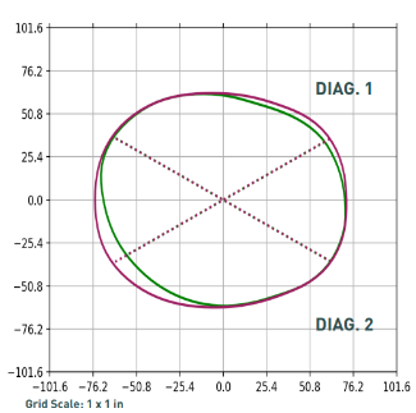
The modified data below, though small, indicates to a clinician when foam should be excavated, if needed.
Post-Treatment Outcomes
In only 47 days (or 6 ½ weeks), this patient’s TDD dropped from 14 to 5.5 millimeters. The CVAI reduced from 9.6 to 3.7. On the CHOA Plagiocephaly Severity Scale, this patient moved from Level 4 to Level 1. Because the patient was meeting developmental milestones, treatment was terminated with the clinical recommendation that the patient undergo a repositioning program.
Follow-Up
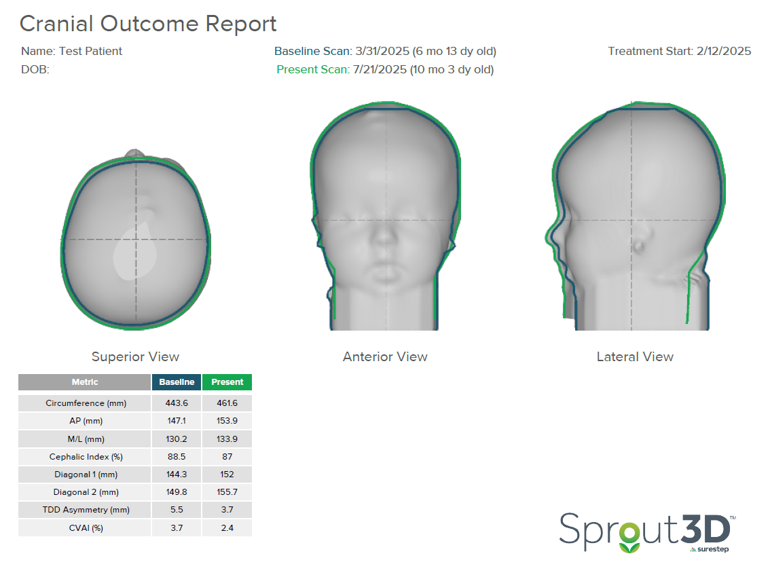
The clinician brought this patient back in for a scan four months after terminating treatment. Through repositioning and meeting general milestones, their TDD further reduced from 5.5 to 3.7 millimeters. Their CVAI dropped from 3.7 to 2.4, which is within normal limits on the CHOA Plagiocephaly Severity Scale.
Case Study 2: Severe Brachycephaly
A four-month-old patient presented with severe brachycephaly. No facial asymmetry or ear misalignment was detected in the initial scan below.
Initial Scan
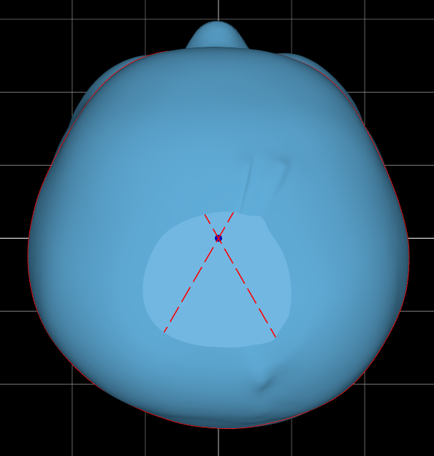
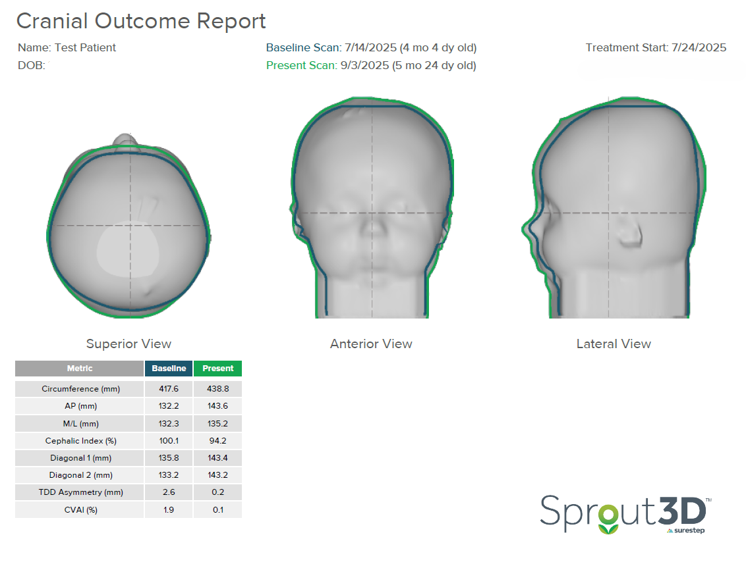
In the initial outcome report below, the severity in the superior transverse plane and lateral view of the head is notable. There is no occipital contour or prominence. This patient was scanned at four months and fit at four and a half months.
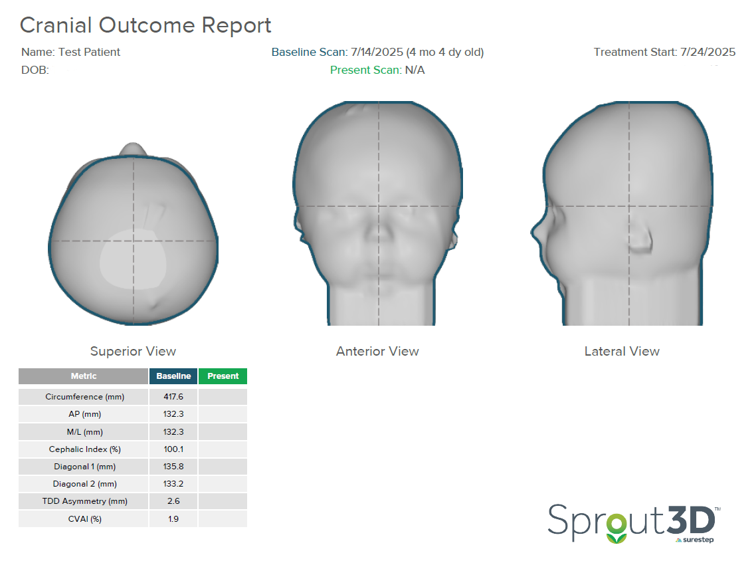
This patient started treatment with a CI of 100.1%. Based on limited brachycephaly severity data, the framework provided in this study was used to assess severity, which suggests that children with a cephalic index greater than 100% with vertical head shape or temporal bossing fall into the severe brachycephalic category. This indicates that CRO treatment should be started.
Treatment Plan
The patient was fit 10 days following this scan. Like the previous case study, the clinician selected standard brachycephaly modifications for this patient.
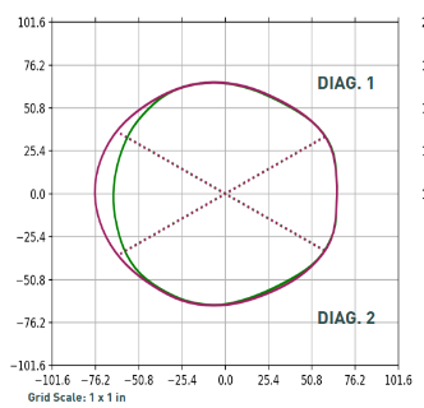
This head shape has been modified down to 92% corrected CI with a strong hold on the euryons to prevent the patient from falling into the posterior void when lying in a supine position.
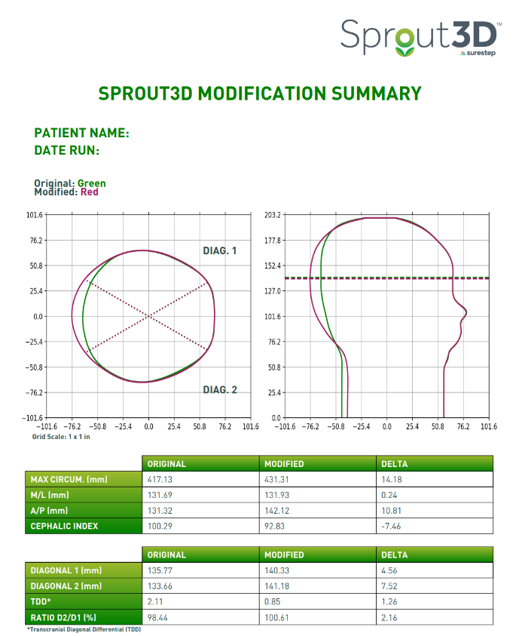
Post-Treatment Outcomes
In only 41 days (just under 6 weeks), this patient’s CI reduced from 100.1% to 94.2%, which means that this patient now falls into the 90-100% or moderate brachycephalic range. They will continue treatment and assess progress in a few weeks.
Clinician & Parent Feedback
The feedback we have received from clinicians has been overwhelmingly positive, with one clinician sharing:
“The Sprout3D is lighter weight, lower profile, and so much easier to put on that most kiddos don’t even react to having it on…With the option of greater ventilation, kids don’t seem to be quite as hot and sweaty while wearing the CRO, which leads to less skin irritation and greater compliance in wearing, resulting in greater outcomes.”
– Greenville, NC
Parents have shared similar feedback around comfort and ease of use:
“When it came down to choosing which helmet I wanted for our son, it was a no-brainer. After hearing about the different options, I chose Sprout3D by Surestep. The helmet is very lightweight and thin, and it was all around a better choice for our family.”
– Springfield, MA
Take the Next Step with Sprout3D
Are you interested in trying Sprout3D with your patients? To get started, sign up for our free onboarding course, where you will learn how to scan, order, and adjust Sprout3D.



